Commercialization kick off ceremony for technology transfer products from Daewoong and first product launching to the market
On December 9th, 2021, Traphaco Joint Stock Company held the Commercialization Kick Off Ceremony for Technology Transfer Products from Daewoong and launched first product to the market, Ursodeoxycholic Acid (UDCA).
Board of Directors representatives and Board of Management attended the Commercialization Kick Off Ceremony for Technology Transfer Products from Daewoong
The Annual General Meeting of Shareholders of Traphaco Joint Stock Company on March 31st, 2021 has set the company's development orientation for the period of 2021-2025, with the goal of total revenue’s compound growth rate reached 13.3%, profit before tax has compound growth rate of 15%, including the important activity of enhancing technology transfer with Traphaco’s partner: Daewoong - Korea with about 70 products, create a breakthrough development in sales of new products.
The technology transfer project for phase 1 has been implemented since the beginning of March 2019, with the following objectives: growing Traphaco's total revenue, increase the proportion of ETC channel's revenue, diversify Traphaco's product portfolio, especially breakthrough non herbal pharmaceuticals products that have the ability to compete in the bidding and prescription channels; improve Traphaco's research and production capacity of non herbal pharmaceutical products with the cooperation and support from Daewoong partner.
Phase 1 has implemented 7 SKUs technology transfer products belonging to 3 treatment groups (hepatobiliary, cardiovascular, gastric); including Ursodeoxycholic Acid (UDCA 100/200/300 mg) with indications for the treatment of gallstones, chronic obstructive hepatitis. Phase 2 has started from August 19th, 2021, includes 12 technology-transferred SKUs; products that ensure the important criteria of: good market potential, highly efficiency in treatment and differentiation, and competitiveness in Vietnam.
Previously, in March 2019, the Kick-off Ceremony of the Phase 1 Technology Transfer Project between Traphaco and Daewoong took place. Right after that, the technology transfer department of the two sides developed a specific plan for the best technology transfer and ensured the progress. In order to have the first batch launching, the two sides have made efforts to speed up the transfer progress within 2 years. From March to September 2019, Traphaco and Daewoong cooperated to carry out the preparation work from equipment, raw materials and production plans... From September to November 2019, Daewoong experts came to Vietnam to transfer the production process according to formulation accurately. The production process follows the accurate formulation and technology in Korea and members regularly keep in touch with Vietnamese manufacturing experts to ensure the best product quality in the following stages.
From November 26th, 2021, the first batch of Acid Ursodeoxycholic 300mg (UDCA) has been shipped to branches nationwide to be ready for sales from December 2021. Acid Ursodeoxycholic (UDCA) has the effect of treating gallstones, chronic obstructive hepatitis, transferred technology with the same quality as Uruso product of Daewoong, a branded product for many years in Vietnam. trusted by doctors and pharmacies; and synchronously deployed on channels: bidding, prescription and OTC channel.
At the Commercialization Kick off Ceremory of technology transfer products from Daewoong and training session of launching the first product batch to the market of Acid Ursodeoxycholic 300 mg (UDCA), Mr. Kim Dong Hyu – Chief of ETC & Business Development Officer said: “Technology transfer is a big project. The project will bring important values to Traphaco; not only in terms of revenue growth, financial performance but also enhancing Traphaco's position for many reasons. Firstly, the cooperation relationship with international partners that is Daewoong company - one of the largest pharmaceutical groups in the Korea market. Secondly, create diversifying products and good product portfolio with many drug groups that have great market demand, newly differentiated technologies, improving research and production capacity of non-herbal medicines of Traphaco. Thirdly, this project will be the beginning step that will start the next big projects of Traphaco in the period of 2021 – 2025”. And Traphaco will not only maintain the leading position of traditional products, but also strongly develop non-herbal medicines in line with current market trends.
Mr. Kim Dong Hyu - Chief of ETC & Business Development Officer speaks at the training session of launching first product batch
Mr. Kim Dong Hyu added that the Acid Ursodeoxycholic 300 mg (UDCA) product marked the first achievement in international cooperation between Traphaco and Daewoong. Products transferred from Daewoong technology are different from those already on the market because they have the same technology, quality and effectiveness as the original products made in Korea. He also hopes that the Sales department will successfully deploy the Acid Ursodeoxycholic 300 mg (UDCA) product and be a premise for Traphaco's next technology transfer products; contributing to the success of the technology transfer project between Traphaco and Daewoong, contributing to the realization of Traphaco's sustainable development orientations.







.JPG)
.JPG)





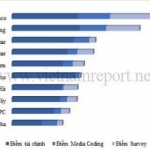








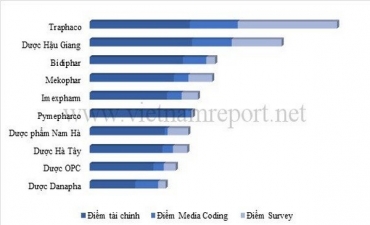

.jpg)
.jpg)




